Tutorialagentsdocument intelligencedatabricksregulatory compliance
Databricks Enables CRL PDF Extraction Pipeline
7.9
Relevance Score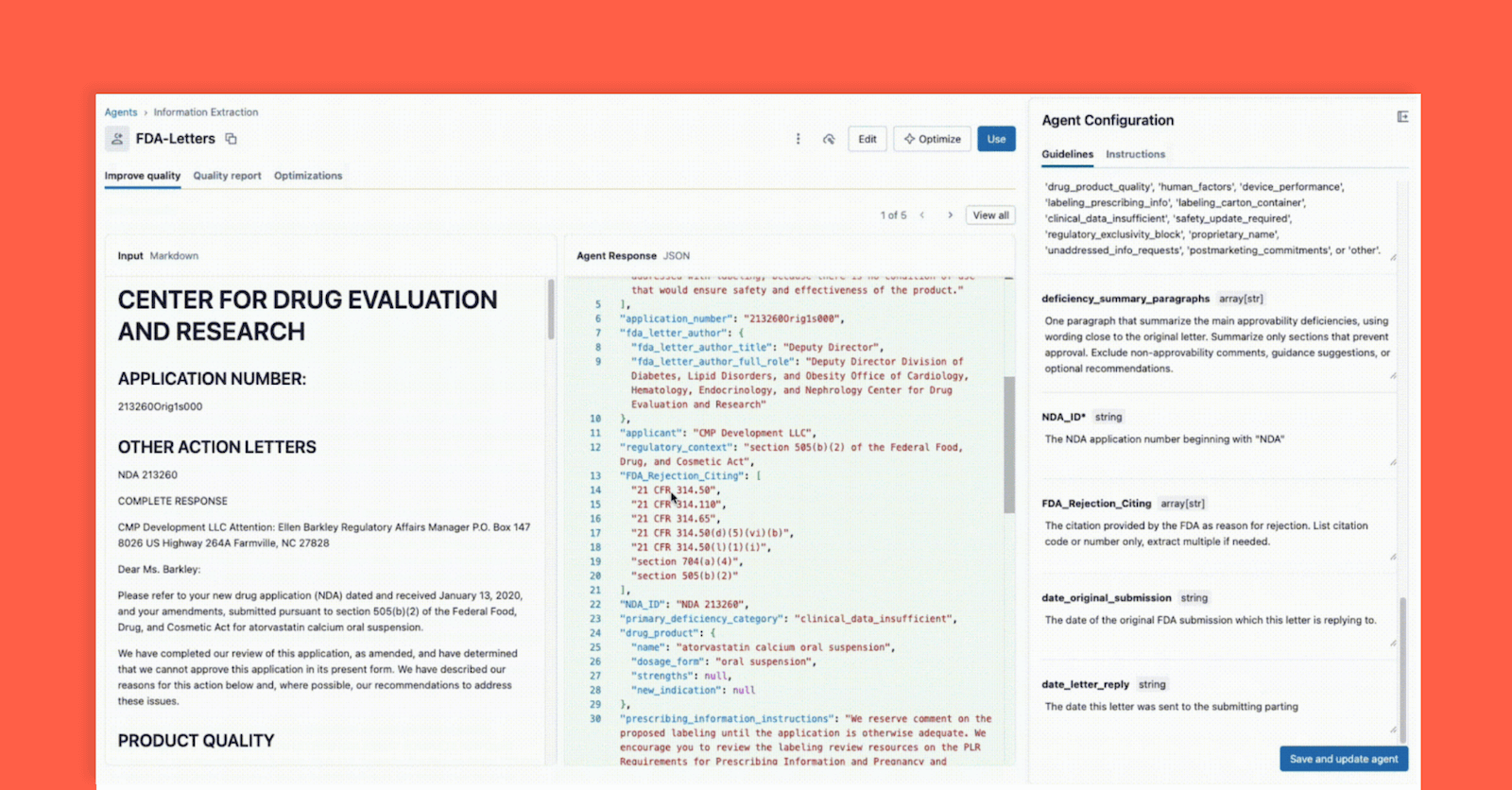
In July 2025 the US FDA released an initial batch of 200+ Complete Response Letters (CRLs) as downloadable PDFs, making regulatory decision rationales publicly accessible. Databricks demonstrates a four-step workflow using ai_parse_document(), Information Extraction Agent Bricks, and evaluation tooling to parse, extract, and validate structured data from CRLs, enabling organizations to derive regulatory intelligence and reduce submission risk.



